Biocon Q3FY24 Revenue at Rs 4,519 Cr, Up 50%; Biosimilars Up 65%; Research Services Up 9%; EBITDA at Rs 1,492 Cr, Up 106%; Net Profit at Rs 660 Cr Pre-Paid USD 200 Mn towards Debt Reduction
-
Thu, 08-Feb-2024
- Posted by: Biocon Biologics
Bengaluru, Karnataka, India: February 8, 2024:
Biocon Ltd (BSE code: 532523, NSE: BIOCON), an innovation-led global biopharmaceuticals company, today announced its consolidated financial results for the fiscal third quarter ended December 31, 2023.
Leadership Comments
BIOCON GROUP
“Biocon delivered Consolidated Revenue of Rs 4,519 crore for Q3FY24, driven by 65% growth in Biosimilars and 9% growth in Research Services. Consolidated EBITDA at Rs 1,492 crore grew by 106%. Net Profit, boosted by other income, stood at Rs 660 crore.
“A key milestone this quarter was the successful conclusion of the transition of the acquired business by Biocon Biologics and a pre-payment of ~USD 200 million towards the acquisition-related debt reduction. Steady market shares for key biosimilars in the U.S. and EU were complemented by the Emerging Markets performance which saw a number of new product launches and tender wins.
“We continue to make steady progress towards strengthening the foundation for a sustainable growth across all three business segments.”
— Kiran Mazumdar Shaw, Executive Chairperson, Biocon and Biocon Biologics
BIOCON GENERICS
“The Generics business delivered 4% sequential revenue growth in the third quarter, driven by higher API sales.
“The year-on-year performance, however, was muted on account of continued pricing pressure that impacted customer offtake in our API business, compared to the previous fiscal. This was partially offset by growth in our Generic Formulations portfolio. While we expect pricing pressure in the API business to persist, we continue our focus on driving cost and execution efficiencies throughout the business, to mitigate future impact.
“We received a tentative approval of our ANDA for Dasatinib tablets from the U.S. FDA recently, which reinforces our strategy to vertically integrate complex, difficult-to-make products.”
— Siddharth Mittal, CEO & Managing Director, Biocon Limited
BIOCON BIOLOGICS
“Biocon Biologics successfully completed the complex, accelerated integration of the entire acquired business across 120+ countries, one year ahead of plan, transforming us into a unique, fully integrated, ‘lab-to market’ leading global biosimilars player. Our focus has been on preserving value, business continuity and ensuring a seamless experience for our patients, customers, and partners across the world. This success is a testament to the hard work and dedication of our team and advisors.
“During the quarter, the business delivered 65% year-on-year growth as we continued to see robust demand for our products with market shares increasing across geographies. We onboarded new customers and won several key tenders.”
— Shreehas Tambe, CEO & Managing Director, Biocon Biologics Limited
SYNGENE
“Syngene reported a revenue growth of 9% year-on-year in the third quarter on the back of good performance by Dedicated Centers, Development and Manufacturing divisions. Though demand in our Discovery Services division was impacted during the quarter by headwinds in the U.S. biotech segment, we are starting to see early signs of funding levels stabilizing. Our business model remains resilient, and we are continuing to invest in scientific capabilities and important enterprise projects as industry fundamentals for pharma outsourcing remain positive for the medium-to-long term.”
— Jonathan Hunt, CEO & Managing Director, Syngene
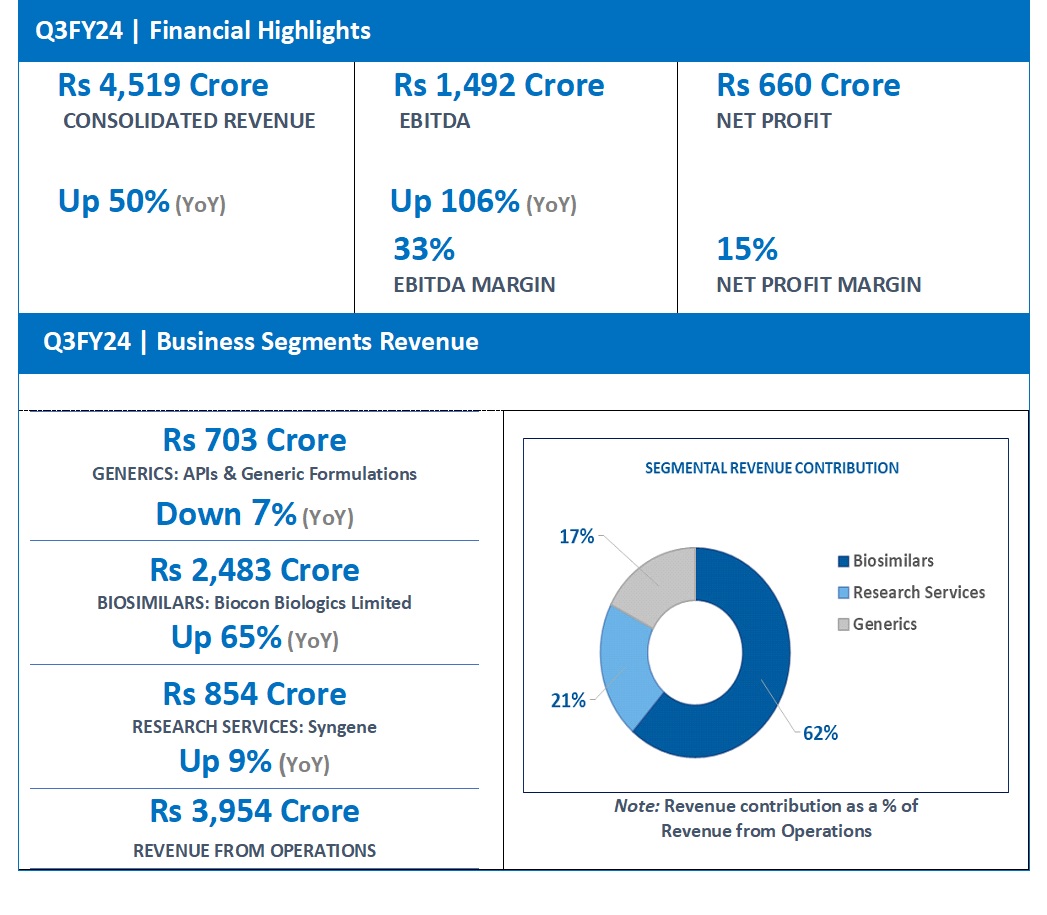
FINANCIAL HIGHLIGHTS (CONSOLIDATED): Q3FY24
In Rs Crore
| Particulars | Q3FY24 | Q3FY23 | YoY (%) |
| INCOME | |||
| Generics | 703 | 760 | – 7% |
| Biosimilars | 2,483 | 1,507 | 65% |
| Novel Biologics | – | – | |
| Research services | 854 | 786 | 9% |
| Inter-segment | -86 | -112 | -23% |
| Revenue from operations# | 3,954 | 2,941 | 34% |
| Other income$ | 565 | 79 | 615 % |
| Total Revenue | 4,519 | 3,020 | 50% |
| Net R&D Expenses | 329 | 337 | -2% |
| Gross R&D Spend | 332 | 365 | -9% |
| EBITDA | 1,492 | 723 | 106% |
| EBITDA Margins | 33% | 24% | |
| Core EBITDA* | 983 | 1,069 | -8% |
| Core EBITDA Margins* | 27% | 36% | |
| PBT (before Exceptional Items^) | 787 | 246 | 220% |
| Net Profit (before Exceptional Items^) | 644 | 140 | 360% |
| Net Profit Margin (before Exceptional Items^) | 14% | 5% | |
| Net Profit^ | 660 | -42 | |
| Net Profit Margin | 15% | -1% |
Figures above are rounded off to the nearest Crore; % based on absolute numbers.
# Includes income from the divesture of two non-core business assets of Biocon Biologics’ Branded Formulations India business amounting to Rs 350 crore and Licensing income.
*Core EBITDA is EBITDA net of R&D expense, licensing, forex, dilution/fair valuation gain in Bicara, sale of non-core BFI assets and mark-to-market movement on investments.
Notes to financials above:
$Other income for Q3FY24 includes a gain of Rs 456 crore mainly from the fair valuation of Biocon’s holding in Bicara Therapeutics, resulting from Bicara’s Series C financing.
^ Exceptional items during Q3 FY24 amount to a net gain of Rs 21 crore. Net of tax and minority interest, exceptional gains during Q3FY24 amounted to Rs 16 crore, resulting in a Net Profit of Rs 660 crore. Please refer to Note 17: Exceptional Items in the published Financial Results.
Financial Commentary: Q3FY24
Consolidated Revenue for Q3FY24 grew 50% year-on-year (YoY) to Rs 4,519 crore. This growth was supported by income from the divesture of two non-core business assets of Biocon Biologics’ Branded Formulations India business amounting to Rs 350 crore and a gain of Rs 456 crore from Biocon’s stake dilution in Bicara Therapeutics.
EBITDA for the quarter increased by 106% to Rs 1,492 crore, representing an EBITDA margin of 33% versus 24% in the same period last year.
Depreciation, amortisation, and interest increased by Rs 260 crore over last year. This is primarily related to the biosimilars business acquisition cost.
Net R&D investments for the quarter were Rs 329 crore, representing 11% of revenue ex-Syngene.
Core EBITDA at Rs 983 crore, represents core operating margins of 27%.
Profit Before Tax and exceptional items stood at Rs 787 crore.
Reported Net Profit for the quarter stood at Rs 660 crore versus a Net Loss of Rs 42 crore in the same quarter of the previous year.
CORPORATE HIGHLIGHTS
Board Announcements
Biocon Biologics Limited
Nicholas Robert Haggar has been appointed as an Independent Director on the Board of Biocon Biologics Limited for a period of three years with effect from February 6, 2024.
He has over 30 years of experience in leading and building pharmaceutical and healthcare enterprises and has been successfully guiding innovation and increasing access to medicines with deep commitment to patients, compliance, quality and sustainability. He has been a part of Biocon Limited’s Board as an Additional Director, since September 2023.
Nicholas has held the position of CEO, Zentiva SA; CEO, Insud Pharma SL; President of Medicines for Europe; and Regional Director, Sandoz. He is currently the CEO & Founder of HealthQube Ltd and a Non-Executive Director of Zentiva.
Management Announcements
Biocon Limited
Nitin Prabhakar Shenoy has been appointed as Head of IT & Digital Transformation, Biocon Limited. Nitin brings with him nearly three decades of experience in IT operations, SAP delivery including support, implementation and pre-sales, assessment and design of MES systems.
Awards & Recognitions
ESG Practices: Biocon (including Biocon Biologics)
- Biocon has been included in the S&P Global Sustainability Yearbook 2024 for the second year in a row, based on its 2023 Corporate Sustainability Assessment.
- The Company has improved its S&P Global ESG Score to 63 from 52 previously.
- Biocon continues to be a member of the DJSI Emerging Markets Indexfor the third year in a row.
- Biocon Biologics has been recognized by the ‘Life at Work Awards’ as one of the Top 3 companies for Sustainability and Diversity, Equity & Inclusion (DEI) in Malaysia.
Intellectual Property Strategy
- Biocon Biologics has been included in the ASIA IP ELITE list for 2023 by IAM (Intellectual Asset Management), the world’s biggest IP publication.
- Biocon Biologics also won the CII’s Special Appreciation IP Award 2023 at the 9th International Conference on Intellectual Property Rights (IPR).
Operational & Quality Excellence
- Biocon won the prestigious Jury Champion Award in the ‘Breakthrough Category’ at the 46th CII National Kaizen Competition and two awards at QCFI’s 37th National Convention on Quality Concept 2023.
- Biocon Biologics won 3 awards in the 4th National Challenger’s Trophy event as part of the 46th CII National Kaizen Competition and one award at QCFI’s 37th National Convention on Quality Concept 2023.
- These awards recognize the best industry practices for quality and operational excellence.
People Practices
- Biocon Biologics has been recognized among the ‘100 Best Companies for Women’ and ‘Top 100 Exemplars of Inclusion’ in India for the sixth time in a row by Avtar & Seramount.
Business Highlights
BIOSIMILARS: Biocon Biologics Limited (BBL)
- Q3FY24 Revenue at Rs 2,483 Crore, up 65% (YoY) from Rs 1,507 Crore in Q3FY23.
- Reduced the acquisition debt by USD 200 million.
- Preserved, consolidated and built business post successful integration.
- Served 5.5+ million patients (MAT December 2023) ##
##12-month moving annual patient population (January 2023 to December 2023)
Business Performance
Biocon Biologics reported a YoY growth of 65% for Q3FY24 with revenue at Rs 2,483 crore, demonstrating its ability to maintain its growth momentum while completing the complex and accelerated integration of the acquired biosimilars business. In this quarter, Biocon Biologics successfully completed its transition to a fully integrated, ‘lab-to-market’ global biosimilars enterprise with a presence across 120+ countries.
During this time of rapid operational transformation, the Company successfully maintained business continuity while seamlessly serving patients, partners, and customers worldwide and securing new customers and tenders in both Advanced and Emerging Markets.
EBITDA for the quarter reported a growth of 98% at Rs 714 crore, representing an EBITDA margin of 29%.
During the quarter, Net R&D investments stood at Rs 265 crore, representing 11% of Biocon Biologics’ revenue for the quarter, reflecting the advancement of our biosimilars pipeline.
Core EBITDA at Rs 587 crore, with Core EBITDA margins at 28%, were impacted on account of a series of transition related expenses and one-off costs.
Biocon Biologics prepaid ~USD 200 million towards reducing the acquisition-related debt during the quarter.
Advanced Markets
North America@
Biocon Biologics sharpened its focus on a self-led commercial model in North America, following the seamless integration of people and business operations.
The Company’s products maintained momentum and showed resiliency in a dynamic market in the immediate quarter post the integration of the acquired biosimilars business. It expanded market access across all therapeutic segments in the U.S., making strides in insulins and oncology portfolios and securing several immunology formulary wins.
Biocon Biologics drove an uptick in sales of unbranded bGlargine with a closed-door pharmacy network even as unbranded bGlargine along with Semglee® held market share steady at ~12%.
Fulphila® (bPegfilgrastim) maintained ~18% market share in the U.S. The Company won three new contracts, including a sole-source contract from a large group purchasing organization (GPO) for the pre-filled syringe presentation of its bPegfilgrastim. Ogivri® (bTrastuzumab), which reported a market share of ~12% in U.S., secured three new contracts, including a large GPO agreement.
In line with the improvement in market adoption of biosimilar Adalimumab in the U.S., Biocon Biologics successfully secured two contracts for its unbranded bAdalimumab, both commencing in February 2024.
Europe and JANZ&
By the end of Q3FY24, Biocon Biologics successfully integrated the acquired biosimilars business across more than 30 countries in Europe as well as Japan, Australia and New Zealand (JANZ).
In Europe, the market share for Fulphila® grew to 8% in Q3. Existing in-market products such as Ogivri®, Abevmy®, and Hulio® held steady market shares at 6% each across the region. In Australia, Ogivri® holds a strong ~16% market share.
The Company has partnered with Sandoz granting it exclusive rights to promote, sell and distribute “Adalimumab BS for subcutaneous injection [FKB]” *** in Japan.
Emerging Markets (EMs)
Following the successful transition of 85+ Emerging Markets to Biocon Biologics, the Company has augmented its focus on nine self-led markets and all other partner-led countries. The Company also onboarded new partners for its products in many countries in APAC, LATAM and AFMET.
In Q3FY24, Biocon Biologics ensured business continuity in both partner- and self-led markets. It pursued new growth avenues by winning several tenders for its products, including for the acquired business in territories like Tunisia, Libya and Ukraine, in addition to obtaining numerous approvals for key biosimilars across EMs.
With the launch of Abevmy® (bBevacizumab), Biocon Biologics has initiated its ‘direct to market’ journey in Brazil, which is the largest market for this molecule in LATAM. Innovator sales for Bevacizumab in Brazil are over USD 175 million^^^. The Company is seeing positive traction for Abevmy® despite the presence of multiple players in the market.
Expanding Insulins Access
Biocon Biologics is addressing the challenge of insulin inequity through its Market Access programs in several countries. In Q3FY24, the Company supplied its bGlargine at subsidized rates for the benefit of ~100 young people with Type 1 diabetes in Myanmar.
The Company also donated 12,500 bGlargine pens and 1,000 vials to the U.S.-based ‘Insulin for Life’, a non-profit organization that provides donated supplies to partner clinics and hospitals. It addresses the needs of patients with diabetes in LMICs (low- and middle-income countries) around the world.
Regulatory & Clinical Updates
Key highlights include initiation of global Phase 3 clinical trials for bPertuzumab and a marketing authorization approval for YESAFILI® (bAflibercept) from MHRA, UK.
@ North America: Market shares based on IQVIA November 2023 data.”
& Europe & JANZ: Market shares based on IQVIA Q3 CY2023 data.
***Fujifilm Kyowa Kirin Biologics Co. Ltd. is a Marketing Authorization Holder in Japan.
^^^Emerging Markets: Bevacizumab Innovator Sales in Brazil; Source: FMB+NRC Reference MAT August 2023.
GENERICS: APIs & Generic Formulations
- Q3FY24 Revenue at Rs 703 Crore down by 7% (YoY) from Rs 760 Crore in Q3FY23.
Business Performance
Performance during the quarter saw sequential revenue growth, led by higher API sales. The subdued year-on-year performance was due to pricing pressure in the API business that impacted customer offtake, which was partially offset by sustained growth across most of the Generic Formulation products.
The Company received its first Generic Formulations approval in China for Mycophenolate Sodium (MPS), an immunosuppressant used to help prevent organ transplant rejection. This paves the way for the Company’s finished dosage formulations foray into China, a key strategic market.
Biocon also received a tentative approval for Dasatinib tablets, for 20 mg, 50 mg, 70 mg, 80 mg, 100 mg, and 140 mg strengths, from the U.S. Food and Drug Administration (FDA), recently. Dasatinib is indicated for use in the treatment of Philadelphia chromosome positive chronic myeloid leukemia in adults. It is also used to treat Philadelphia chromosome positive acute lymphoblastic leukemia in adults with resistance or intolerance to prior therapy.
TGA Australia completed an inspection of the Company’s API facility in Visakhapatnam with no critical observations. The API facility at Hyderabad also underwent a remote inspection by Cofepris, Mexico, with two observations, for which responses have been submitted.
Post completion of process validation at the Company’s greenfield immunosuppressants facility in Visakhapatnam, the site obtained a Certification of Suitability (CEP) from the European Directorate for the Quality of Medicines (EDQM). The Company expects the site to be inspected and qualified by other regulatory authorities during the next fiscal, enabling commercialization of its products across geographies.
Process validation at the Company’s large volume peptides facility at Bengaluru was successfully completed during the quarter. At the Synthetic API facility in Hyderabad, process validation was initiated.
NOVEL BIOLOGICS
Biocon’s Boston-based associate Bicara Therapeutics closed a USD 165 million Series C financing in December 2023, led by TPG and Braidwell. With this, USD 355 million has been raised to date from a syndicate of biotech investors, with all Series B investors participating in this round of financing.
As a result, the Company has recorded a gain of Rs 456 crore, arising from dilution of Biocon’s shareholding to 14% and loss of significant influence over Bicara. Hence it will no longer be an ‘associate company’ of the Biocon Group.
RESEARCH SERVICES: Syngene
- Q3FY24 Revenue at Rs 854 Crore, up 9% (YoY) from Rs 786 Crore in Q3FY23.
Business Performance
In Q3FY24, the Research Services business delivered positive performances in Development and Manufacturing Services divisions as well as in the Dedicated Centers. Syngene’s performance in Discovery Services was impacted by the slowdown of funding in the global biotech ecosystem. In Manufacturing Services, the Company continued to make good progress on the long-term biologics manufacturing partnership with Zoetis. Overall, the Company maintained healthy EBITDA margins of 30%.
The acquisition of the biologics facility from Stelis Biopharma Ltd has been completed. Once operational, it will add to both drug substance and drug product capabilities of Syngene.
The Company also added a biologics assay capability at the Hyderabad campus to complement the existing Drug Metabolism and Pharmacokinetics (DMPK) and Compound Management facilities.7
Enclosed: Fact Sheet – with Financials as per IND-AS
About Biocon Limited:
Biocon Limited, publicly listed in 2004, (BSE code: 532523, NSE Id: BIOCON, ISIN Id: INE376G01013) is an innovation-led global biopharmaceuticals company committed to enhance affordable access to complex therapies for chronic conditions like diabetes, cancer and autoimmune. It has developed and commercialized novel biologics, biosimilars, and complex small molecule APIs in India and several key global markets as well as Generic Formulations in the US, Europe & key emerging markets. It also has a pipeline of promising novel assets in immunotherapy under development. Website: www.biocon.com; Follow-us on Twitter: @bioconlimited for company updates.
Biocon Biologics Limited (BBL), a subsidiary of Biocon Ltd., is a unique, fully integrated, global biosimilars company committed to transforming healthcare and transforming lives by enabling affordable access to high quality biosimilars for millions of patients worldwide. It is leveraging cutting-edge science, innovative tech platforms, global scale manufacturing capabilities and world-class quality systems to lower costs of biological therapeutics while improving healthcare outcomes. BBL has integrated the acquired global biosimilars business of its long-standing partner Viatris, which is a historic milestone in its value creation journey. Biocon Biologics has commercialized eight biosimilars in key emerging markets and advanced markets like U.S., Europe, Australia, Canada, and Japan. The Company has a pipeline of 20 biosimilar assets across diabetology, oncology, immunology, ophthalmology, and other non-communicable diseases. It has many ‘firsts’ to its credit in the biosimilars industry. As part of its environmental, social and governance (ESG) commitment, BBL is advancing the health of patients, people, and the planet to achieve key UN Sustainable Development Goals (SDGs). Website: www.bioconbiologics.com; Follow us on Twitter: @BioconBiologics and LinkedIn: Biocon Biologics for company updates.
 Brazil
Brazil Egypt
Egypt Europe
Europe Hong Kong
Hong Kong Malaysia
Malaysia Mexico
Mexico Morocco
Morocco Philippines
Philippines Saudi Arabia
Saudi Arabia South Africa
South Africa Taiwan
Taiwan Thailand
Thailand Tunisia
Tunisia Turkey
Turkey UAE
UAE USA
USA Vietnam
Vietnam Global HQ
Global HQ
