Biocon Q1FY25 Revenue up 30% at Rs 4,567 Cr EBITDA up 117% at Rs 1,755 Cr Net Profit at Rs 660 Cr
-
Thu, 08-Aug-2024
- Posted by: Biocon Biologics
Bengaluru, Karnataka, India: August 8, 2024:
Biocon Limited (BSE code: 532523, NSE: BIOCON), an innovation-led global biopharmaceuticals company, today announced its consolidated financial results for the fiscal first quarter ended June 30, 2024.
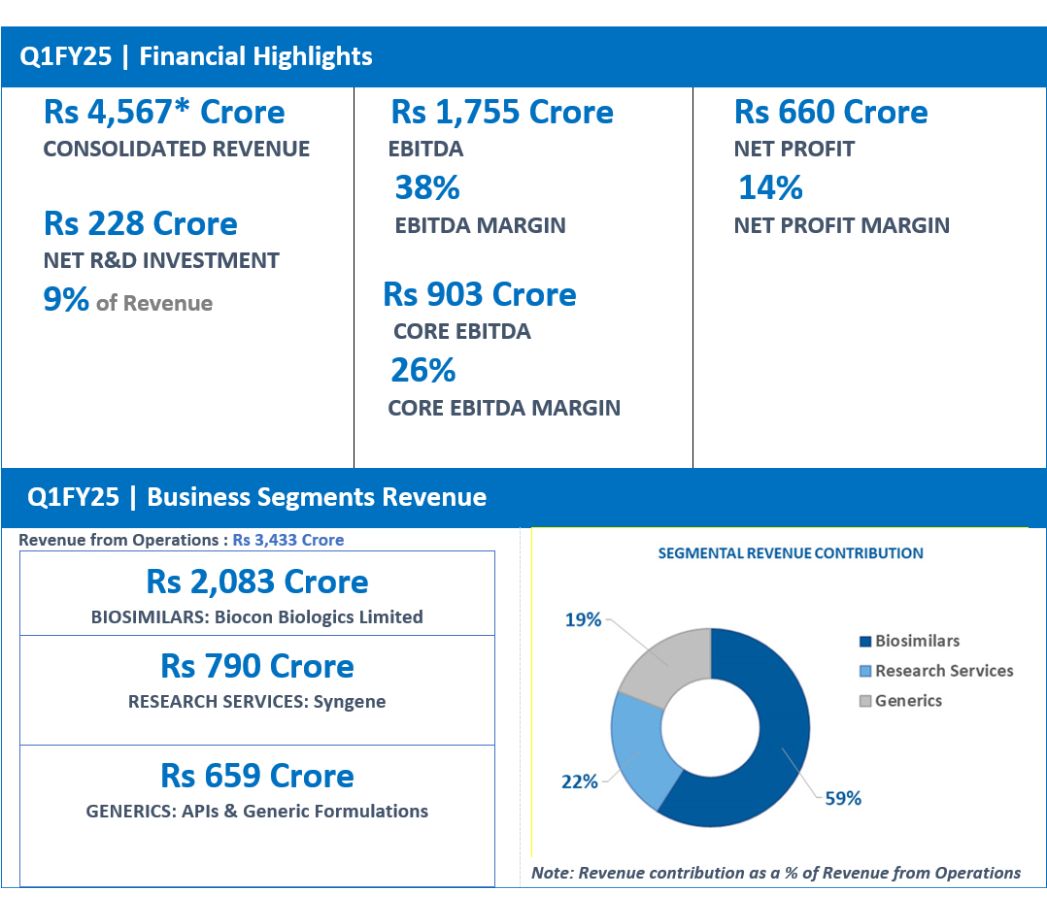
Note: *Q1FY25 Consolidated Revenue includes proceeds of Rs 1057 Cr on account of the strategic collaboration between Biocon Biologics & Eris Lifesciences.
Leadership Comments
BIOCON GROUP
“Biocon has reported consolidated revenues of Rs 4,567 crore for Q1FY25, delivering a strong 30% YoY growth, with EBITDA growing 117% to Rs 1,755 crore and a Net Profit of Rs 660 crore. This strong performance was primarily on account of a one-time gain from the strategic collaboration between Biocon Biologics and Eris Lifesciences. The underlying business performance of Biocon has been in line with our expectations. Post integration, the Biosimilars business has delivered a healthy performance with 11% like- for- like growth, as it consolidates business across global markets. This has helped offset the challenges of pricing pressures in the Generics segment and the difficult U.S. Biotech funding environment, which has impacted the growth trajectory of our Research Services business.
“The outlook for this fiscal remains positive as we anticipate stronger growth in H2FY25, with new product launches in the Biosimilars and Generics businesses, including Liraglutide for diabetes and obesity in the UK and other markets. Additionally, we expect improved business prospects for Syngene, supported by a resurgent biotech funding environment in the U.S.”
— Kiran Mazumdar-Shaw, Executive Chairperson, Biocon and Biocon Biologics.
BIOCON GENERICS
“The Generics business continued to encounter pricing pressure and demand contraction, which impacted our first quarter’s performance of both APIs and Generic Formulations.
The business made progress on its Most of the World (MoW) expansion strategy with the signing of a partnership agreement with Handok in South Korea for the commercialization of Liraglutide. We also obtained our first injectable approval in the U.S. for Micafungin and secured some key customer contracts in the region.
“Looking ahead, our focus will be directed towards commercializing products for which we have received approvals, including Liraglutide, in the second half of this fiscal, as indicated previously. We will also continue to accelerate our cost improvement initiatives and expedite on-going capex projects.”
— Siddharth Mittal, CEO & Managing Director, Biocon Limited.
BIOCON BIOLOGICS
“Having successfully integrated the acquired business last year, Biocon Biologics’ focus in FY25 now shifts to consolidation and setting up the business for growth. We began the fiscal year on a strong note with the business delivering year-on-year growth of 11% on a like-for-like basis. This growth was underpinned by an increase in market shares, tender wins in Europe and Emerging Markets and 15 new product launches.
This quarter YESAFILI™, our bAflibercept, became the 1st interchangeable biosimilar to be approved by the U.S. FDA. Our manufacturing facilities in Bengaluru, India and Johor, Malaysia received GMP certifications from leading regulatory agencies such as the European Medicines Agency (EMA) and Therapeutic Goods Administration (TGA), Australia. These milestones will serve as growth catalysts and allow us to expand our reach to millions of patients globally.”
— Shreehas Tambe, CEO & Managing Director, Biocon Biologics Limited.
SYNGENE
“First quarter performance was broadly flat, in line with our expectations, reflecting the dip in funding for U.S. biotechs that has impacted our sector over the last two years. However, the value of U.S. biotech funding has seen a marked improvement in the first half of 2024. It will take a while for this funding to flow through into outsourcing activities and Syngene is in a strong position to capture a significant share of the upturn in biotech spending in the months ahead.”
—Jonathan Hunt, CEO & Managing Director, Syngene International Limited.
FINANCIAL HIGHLIGHTS (CONSOLIDATED): Q1FY25
In Rs Crore
| Particulars | Q1FY25 | Q1FY24 | YoY (%) |
| INCOME | |||
| Generics | 659 | 700 | -6 |
| Biosimilars^ | 2,083 | 2,015 | 3^ |
| Research services | 790 | 808 | -2 |
| Inter-segment | (100) | (101) | -1 |
| Revenue from operations | 3,433 | 3,423 | 0 |
| Other Income* | 1,134 | 94 | 1,113 |
| Total Revenue | 4,567 | 3,516 | 30 |
| Net R&D Expenses | 228 | 315 | -28 |
| Gross R&D Spend | 228 | 324 | -30 |
| EBITDA | 1,755 | 808 | 117 |
| EBITDA Margins | 38% | 23% | |
| Core EBITDA** | 903 | 936 | -4 |
| Core EBITDA Margins | 26% | 28% | |
| PBT | 1,146 | 184 | 524 |
| Net Profit (before Minority Interest ) | 862 | 149 | 479 |
| Net Profit | 660 | 101 | 551 |
Figures above are rounded off to the nearest Crore; % based on absolute numbers.
Notes to financials above:
^Biosimilars Business revenue grew by 11% on a like- for- like basis.
* Q1FY25 includes proceeds of Rs 1057 Cr on account of the strategic collaboration between Biocon Biologics & Eris Lifesciences, and is reported under Other Income
**Core EBITDA excludes proceeds from the Eris transaction, R&D, Forex, Licensing income, Bicara fair value gain and derivative MTM loss/ gain
Financial Commentary: Q1FY25
Total Consolidated Revenue for Q1FY25 grew 30% year-on-year (YoY) to Rs 4,567 crore.
Other Income includes proceeds of Rs 1,057 crore on account of the strategic collaboration between Biocon Biologics and Eris Lifesciences.
Core EBITDA at Rs 903 crore, represents core operating margins of 26%.
Net R&D investments for the quarter were Rs 228 crore, representing 9% of revenue ex-Syngene.
EBITDA for the quarter stood at Rs 1,755 crore, representing an EBITDA margin of 38%.
Profit Before Tax and exceptional items stood at Rs 1,114 crore.
Net Profit for the quarter, before exceptional items, stood at Rs 648 crore.
Net Profit (before minority interest) for the quarter was Rs 862 crore.
Reported Net Profit for the quarter stood at Rs 660 crore.
CORPORATE HIGHLIGHTS
Management Updates
Biocon Biologics Limited
Joe Sian Chean Fei has been appointed as Vice President & Site Head, at Malaysia, to provide strategic leadership and direction to the site and oversee day-to-day operations across all functions. He brings with him over 25 years of experience in the Malaysian pharmaceutical industry and has a successful track record of managing pharmaceutical operations, strategic planning, and organizational development.
Business Highlights
GENERICS: APIs & Generic Formulations
- Q1FY25 Revenue at Rs 659 Crore, down 6% YoY
Business Performance
The Generics business reported a subdued first-quarter performance with demand challenges on its base products impacting revenues.
During the quarter, the Company obtained its first injectable approval in the U.S. – Micafungin for injection in 50mg and 100mg strengths. Micafungin is an antifungal medicine used to treat or prevent certain kinds of fungal or yeast infections.
The business also secured new customer contracts for Mycophenolic Sodium tablets and Liothyronine Sodium tablets in the U.S.
In line with the business’ regional expansion strategy, an exclusive licensing and supply agreement was entered into with Handok, a specialty pharmaceutical company in South Korea, for the commercialization of its vertically integrated drug product, Synthetic Liraglutide, used in the treatment of chronic weight management, as an adjunct to a reduced-calorie diet and increased physical activity.
Regulatory Inspections
The Company received a GMP certificate from TGA, Australia, for its Visakhapatnam API facility (Site 5). A regulatory inspection by ANVISA, Brazil, of its oral solid dosage facility in Bengaluru concluded with no major or critical observations. Two separate GMP inspections of the Company’s API facilities (Sites 5 & 6) at Visakhapatnam by the U.S. Food and Drug Administration (FDA) concluded with four and three observations, respectively, to which the Company has responded with a Corrective and Preventive Action (CAPA) plan. An Establishment Inspection Report (EIR) with Voluntary Action Indicated (VAI) has been received for Site 5 and agency feedback for Site 6 is awaited.
BIOSIMILARS: Biocon Biologics
- Q1FY25 Revenue at Rs 2,083 Crore, Up 11% YoY on a like -for- like basis
- Served 5+ million patients (MAT June 2024 basis)##
##12-month moving annual patient population (July 2023 to June 2024)
Business Performance
Q1FY25
Biocon Biologics’ Q1FY25 revenue* at Rs 2,083 crore reported a YoY growth of 11% on a like- for- like basis after adjusting** Q1FY24 revenue for Branded Formulations, India business.
Core EBITDA stood at Rs 614 crore, with Core EBITDA margin at 30%. EBITDA* at Rs 474 crore reported healthy EBITDA margins of 23%.
Note: *Q1FY25: Revenue and EBITDA does not include proceeds of Rs 1,057 core on account of the strategic collaboration and business transfer to Eris Lifesciences which is booked as Other Income. **Q1FY24 Revenue included Branded Formulations India sales which is not a part of Q1FY25 revenue.
Advanced Markets
North America@
Biocon Biologics is witnessing strong demand for its oncology portfolio, which is driving the growth in the U.S. market. The market share for Ogivri® (bTrastuzumab) grew significantly to 19% from 11% just a year ago. The share of Fulphila® (bPegfilgrastim) increased to 20% from 16% a year ago. Market access agreements for Fulphila® secured earlier came into effect this quarter, contributing to growth. The Company also signed new agreements for Fulphila®, one of which is with a regional health plan.
Biocon Biologics witnessed increased demand for its insulin products with additional market access agreements for bGlargine secured earlier, coming into effect in Q2FY25. The market share of unbranded bGlargine and Semglee® stood at 14%, including share from a large closed-door pharmacy network.
In case of Hulio® (bAdalimumab), a new non-exclusive agreement with a national pharmacy benefit manager (PBM) became operational during the quarter. Additionally, Costco has added unbranded Adalimumab-fkjp Injection for its members in the Costco Members Prescription Program (CMPP), making it available for purchase for all 70 million Costco card holders in the U.S.
In Canada, there were market share improvements across most products over the comparable quarter last year. The share of Hulio® increased to 8% from 6% a year ago, and Kirsty® (bAspart) secured a 3% market share.
During the quarter, the Company received U.S. Food and Drug Administration (FDA) approval for its first- to- file application for YESAFILI™ (Aflibercept-jbvf), an interchangeable biosimilar, which marks Biocon Biologics’ entry into the ophthalmology therapeutic area in the U.S. following approvals in Europe (Sep 2023) and the UK (Nov 2023) where it was the first bAflibercept to be approved.
Europe& and JANZ
The Company is focusing on expansion in Europe and establishing country-level infrastructure in several countries. It improved market shares for some of its products in several key markets and launched existing products in countries where its partner did not have a commercial presence previously.
Hulio® continues to hold leading markets share in France and Germany at ~11% and ~18%, respectively, leading to an overall market share of ~6% in Europe.
During the quarter, the Company secured its first tenders for Abevmy® (bBevacizumab) and Ogivri® in the UK and a few new tenders in Italy, besides expanding its hospital reach in Portugal.
The Company continues to consolidate its business in Japan, Australia, and New Zealand and is exploring meaningful collaborations to drive growth.
@ North America: Market shares based on IQVIA June 2024 data.
& Europe: Market shares based on IQVIA Q1 CY2024 data.
Emerging Markets
Biocon Biologics reported a strong performance in the Emerging Markets on the back of higher sales of its key products bBevacizumab, rh-Insulin and bEtanercept in AFMET; rh-Insulin and bTrastuzumab in APAC; and Glargine, rh-Insulin and bEtanercept in LATAM. This is reflective of the strong sustainable foundation we have built, increase in the underlying demand and patient confidence in our high- quality products. During the quarter, the company expanded its reach by leveraging its new self-commercialization infrastructure and through 12 new launches, in several key markets.
The Company won several tenders for its key products and also secured 14 new regulatory approvals during the quarter, which will pave the way for future growth.
Regulatory Inspections
In June, Biocon Biologics received approval from the European Medicines Agency (EMA) to manufacture bBevacizumab at its world-class monoclonal antibodies Drug Substance facility in Bengaluru. Additionally, EMA renewed its Good Manufacturing Practice (GMP) Certificates of Compliance for the facilities in Bengaluru and Malaysia. In July the Company also received GMP certification from TGA, Australia for bTrastuzumab and bBevacizumab for its new monoclonal antibodies Drug Substance facility at Bengaluru.
Biocon Biologics underwent a U.S. FDA inspection of its manufacturing facilities in Bengaluru in July 2024. The 10-day-long combined cGMP and Pre-Licensing Inspection (PLI) included five monoclonal antibody products. The observations are procedural in nature and will be addressed through a comprehensive Corrective and Preventive Action (CAPA) plan, expeditiously. The Company does not expect the current supply of its products to be impacted.
Scientific Publications
Biocon Biologics continued to contribute to scientific excellence and biopharmaceutical research as evidenced by its recent scientific publications in reputed journals.
-
Use of Biosimilars in LMICs in GaBI Journal
This review article by Biocon offers insights into the challenges of biosimilar uptake and gives recommendations for faster and better access to biosimilars in emerging markets and, more broadly, LMICs. While acknowledging the fact that affordability remains a primary factor for purchasing decisions of biologics, this paper offers additional criteria beyond price that may help the healthcare industry and regulatory agencies in LMICs select quality-assured biosimilars.
-
Aligning Environmental, Social, and Governance to Clinical Development: Moving Towards More Sustainable Clinical Trials in GaBI Journal
This article shows how aligning ESG principles with clinical development can lead to more sustainable clinical trials for biosimilars. The article also talks about developing tools capable of measuring the carbon footprint of biosimilar clinical trials and coming up with guidelines on how to reduce it.
- Comparison of the efficacy and safety of rapid-acting insulin analogs, lispro versus aspart, in the treatment of diabetes: a systematic review of randomized controlled trials in Expert Opinion on Biological Therapy
This review article talks about the potential move from one rapid-acting insulin analog to another, or their biosimilars, to aid better and faster decisions for diabetes management. This systematic literature review demonstrates that both aspart and lispro are comparable in safety and efficacy in patients with type 1 and type 2 diabetes and can be used interchangeably.
Link: https://www.tandfonline.com/doi/full/10.1080/14712598.2024.2371046
RESEARCH SERVICES: Syngene
- Q1FY25 Revenue at Rs 790 Crore, down 2% YoY
Business Performance
The Dedicated Centers and Biologics Manufacturing Services reported steady growth. The repurposing of the biologics manufacturing facility acquired from Stelis Biopharma remains on schedule. Once operational, the facility will triple Syngene’s biologics manufacturing capacity. Though the Discovery Services revenue was hit by the dip in U.S. biotech funding, several pilot projects initiated this quarter will build a foundation for large-scale future collaborations in H2FY25. Development Services continued to attract repeat business from existing clients, reflecting high standards of service delivery.
Enclosed: Fact Sheet – with Financials as per IND-AS
About Biocon Limited:
Biocon Limited, publicly listed in 2004, (BSE code: 532523, NSE Id: BIOCON, ISIN Id: INE376G01013) is an innovation-led global biopharmaceuticals company committed to enhance affordable access to complex therapies for chronic conditions like diabetes, cancer and autoimmune. It has developed and commercialized novel biologics, biosimilars, and complex small molecule APIs in India and several key global markets as well as Generic Formulations in the U.S., Europe & key emerging markets. It also has a pipeline of promising novel assets in immunotherapy under development. Website: www.biocon.com; Follow-us on Twitter: @bioconlimited for company updates.
Biocon Biologics Limited, a subsidiary of Biocon Limited, is a unique, fully integrated, global biosimilars company committed to transforming healthcare and transforming lives. It is capitalizing on its ‘lab to market’ capabilities to serve millions of patients across 120+ countries by enabling affordable access to high quality biosimilars. The Company is leveraging cutting-edge science, innovative tech platforms, global scale manufacturing capabilities and world-class quality systems to lower costs of biological therapeutics while improving healthcare outcomes.
Biocon Biologics has commercialized eight biosimilars in key emerging markets and advanced markets like U.S., Europe, Australia, Canada, and Japan. It has a pipeline of 20 biosimilar assets across diabetology, oncology, immunology, ophthalmology, and other non-communicable diseases. The Company has many ‘firsts’ to its credit in the biosimilars industry. As part of its environmental, social and governance (ESG) commitment, it is advancing the health of patients, people, and the planet to achieve key UN Sustainable Development Goals (SDGs). Website: www.bioconbiologics.com; Follow us on Twitter: @BioconBiologics and LinkedIn: Biocon Biologics for company updates.
Syngene International Ltd. (BSE: 539268, NSE: SYNGENE, ISIN: INE 398R01022) is an integrated research, development, and manufacturing services company serving the global pharmaceutical, biotechnology, nutrition, animal health, consumer goods, and specialty chemical sectors. Syngene’s more than 5600 scientists offer both skills and the capacity to deliver great science, robust data security, and world class manufacturing, at speed, to improve time-to-market and lower the cost of innovation. With a combination of dedicated research facilities for Amgen, Baxter, and Bristol-Myers Squibb as well as 2.2 Mn sq. ft of specialist discovery, development, and manufacturing facilities, Syngene works with biotech companies pursuing leading-edge science as well as multinationals, including GSK, Zoetis and Merck KGaA. For more details, visit www.syngeneintl.com For the Company’s latest Environmental, Social, and Governance (ESG) report, visit https://esgreport.syngeneintl.com/
 Brazil
Brazil Egypt
Egypt Europe
Europe Hong Kong
Hong Kong Malaysia
Malaysia Mexico
Mexico Morocco
Morocco Philippines
Philippines Saudi Arabia
Saudi Arabia South Africa
South Africa Taiwan
Taiwan Thailand
Thailand Tunisia
Tunisia Turkey
Turkey UAE
UAE USA
USA Vietnam
Vietnam Global HQ
Global HQ
