
Biocon Biologics in Mexico
Biocon Biologics is a unique, fully integrated, global biosimilars company with world-class ‘lab-to-market’ capabilities across development, manufacturing, and marketing. It is present in over 120 countries, including over 80 Emerging Market countries, where it is providing sustainable solutions for a healthier world through its cost-effective, high-quality biosimilars.
Expanding Access to Lifesaving Therapies in LATAM
In the Latin America (LATAM) region, Biocon Biologics is making a significant impact on patients’ lives by expanding access to lifesaving treatments and life-improving therapies to treat diseases like diabetes, cancer, and autoimmune conditions through its 8 commercialized biosimilars.
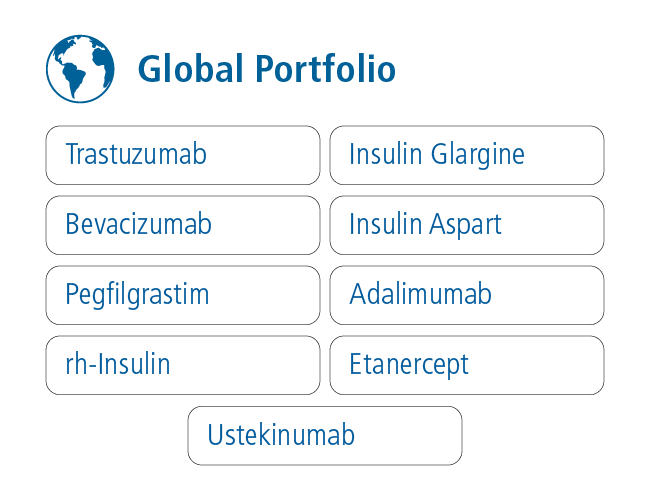
Biocon Biologics has developed a differentiated portfolio of biosimilars spanning insulins, monoclonal antibodies and conjugated recombinant proteins by leveraging its deep experience in biotechnology. It addresses the needs of patients in the LATAM region through its state-of-the-art manufacturing infrastructure, which includes facilities for producing monoclonal antibodies in Bengaluru, India, and insulins in Johor, Malaysia.
Focused on Making a Meaningful Difference in Mexico
In Mexico, Biocon Biologics is focused on broadening access to biosimilars for treating cancer, diabetes and autoimmune disease for the benefit of patients and improving healthcare outcomes in Mexico. Biocon Biologics has three commercialized products in Mexico— Krabeva, a biosimilar to Avastin (Bevacizumab), used in the treatment of various cancers, including colorectal, lung, and kidney cancers, Basalog, a biosimilar to Lantus (Insulin Glargine), a long-acting insulin used to manage blood sugar levels in people with diabetes, and Insugen, a biosimilar to human insulin, used for the treatment of diabetes.
Headquartered in Bengaluru, global biosimilars leader Biocon Biologics has a longstanding presence in the industry, having invested over $1 billion in R&D and global-scale manufacturing over the past two decades. The integration of the global biosimilars business acquired from its long-term partner has further strengthened Biocon Biologics’ commercial presence in Mexico. As a trusted and reliable company, Biocon Biologics is poised to grow and consolidate its leadership in biosimilars in Mexico, making a meaningful impact on patients’ lives.
In Mexico, Bevacizumab is commercialized by our partner Synthon Mexico, and Insulin Glargine and Rh-Insulin are commercialized by our partner Laboratios PISA.
COMMERCIALIZED PRODUCTS
| Sl. No | Brand Name | Molecule Name | THERAPY AREA |
| 1 | Krabeva | Bevacizumab* | Cancer |
| 2 | Basalog | Insulin Glargine** | Diabetes |
| 3 | Insugen | Rh-Insulin** | Diabetes |
*Commercialized by our partner Synthon Mexico in Mexico
**Commercialized by our partner Laboratios PISA in Mexico
Meet The Leaders
Delivering Highest-Quality Products Globally

OUR VISION
To be a global leader in biologics, delivering affordable access to innovative and inclusive healthcare solutions, transforming patients’ lives.

OUR VALUES
Value creation through innovation and differentiation
Quality through compliance and best practices
Collaboration, teamwork and mutual respect
Integrity and ethical behaviour
Performance-driven work culture
Quick Facts
9 biosimilars commercialized in markets globally
11 biosimilars in pipeline
~7.3 billion doses of insulin supplied globally since 2004.
~5 million/year patients served globally
* Number of patients is calculated based on volume supplied
The Biocon Biologics Advantage

Patient-Centricity
Dedicated to expanding patient reach and generating significant savings for patients, payers, and healthcare systems.

Lab-to-Market Expertise
Fully integrated from biosimilars development to manufacturing, distribution, and commercialization.

Legacy of Success
Achieved several industry firstswith U.S. approvals for biosimilar Trastuzumab, biosimilar Pegfilgrastim, and interchangeable biosimilar Insulin Glargine.

Global Scale Production
Operates three large-scale, globally compliant biosimilars manufacturing facilities, ranking among the Top 15* companies worldwide in biomanufacturing capacity.
*19th Annual Report of BioPlan Associates

Wide Commercial Footprint
Commercialized products in 120+ countries through a combination of direct presence, strategic partnerships, and distributors.

High Quality & Compliance Standards
Manufacturing facilities have received 80+ cGMP approvals from over 25 agencies, including the U.S. FDA and EMA.
Business Partnership
Contact Us
Biocon Biologics Limited
Corporate Headquarters
Biocon Biologics Limited
Biocon House, Semicon Park
Electronics City, Phase – II, Hosur Road
Bengaluru 560100, Karnataka, India
Media Contact
Seema Ahuja
Global Head of Corporate Brand & Head
of Communications – EMs
E: Seema.ahuja@biocon.com
 Brazil
Brazil Egypt
Egypt Europe
Europe Hong Kong
Hong Kong Malaysia
Malaysia Mexico
Mexico Morocco
Morocco Philippines
Philippines Saudi Arabia
Saudi Arabia South Africa
South Africa Taiwan
Taiwan Thailand
Thailand Tunisia
Tunisia Turkey
Turkey UAE
UAE USA
USA Vietnam
Vietnam Global HQ
Global HQ





