LEVERAGING THE 'POWER OF ONE'; ENABLING AFFORDABLE ACCESS TO LIFESAVING BIOSIMILARS, WORLDWIDE
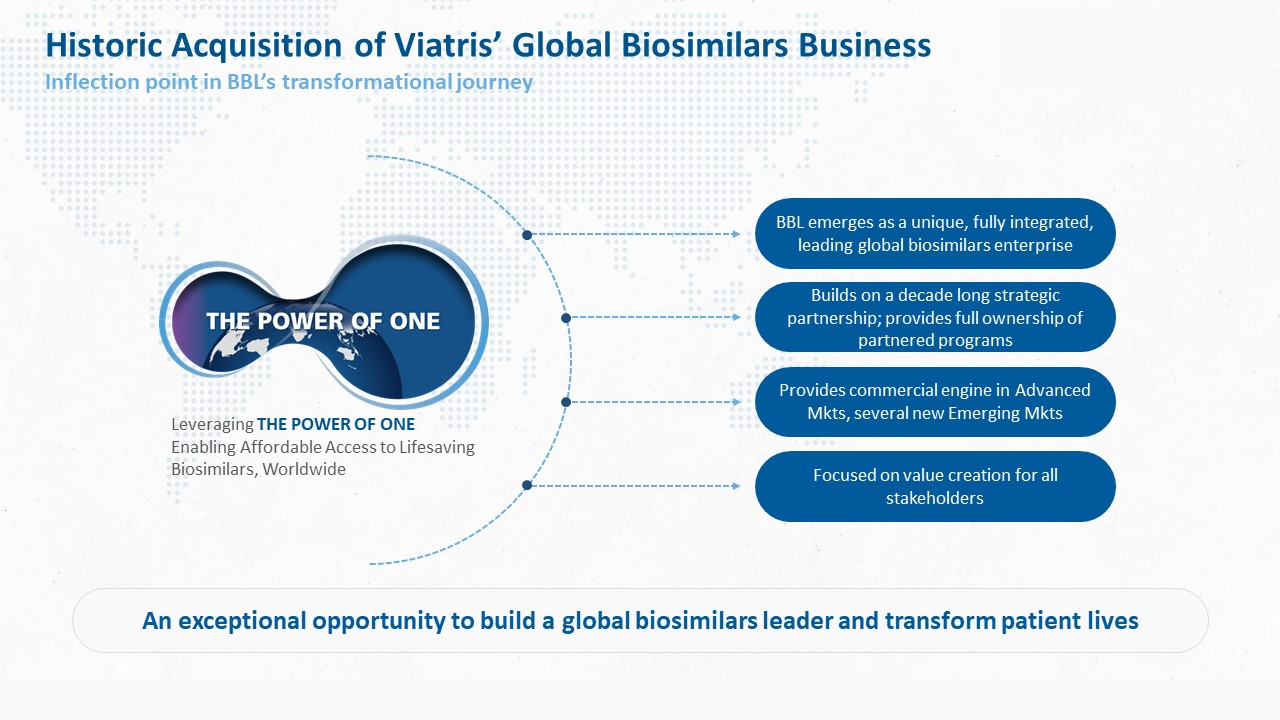
Biocon Biologics has completed a transformative acquisition of the global biosimilars business of its long-term partner Viatris in a cash-and-stock deal worth over USD 3 billion. It positions the company as a leading vertically integrated global biosimilars company. One of the biggest M&A deals in the Indian pharma space, this is a historic inflection point in Biocon Biologics’ value creation journey.
This acquisition is unique as it brings together the two companies’ teams, which have been collaborating for over a decade on projects, into a single, integrated organization driven by a common vision and mission. It strengthens Biocon Biologics’ commitment to expand affordable access to lifesaving biosimilars worldwide, thus addressing global health inequities.
A Transformative Acquisition
The acquisition deal enables Biocon Biologics with direct presence in the advanced markets of U.S., Canada, EU, Australia and New Zealand, in addition to several emerging markets. By providing Biocon Biologics with direct commercial capabilities and supporting infrastructure in these markets, it brings Biocon Biologics closer to patients, customers, and payors.
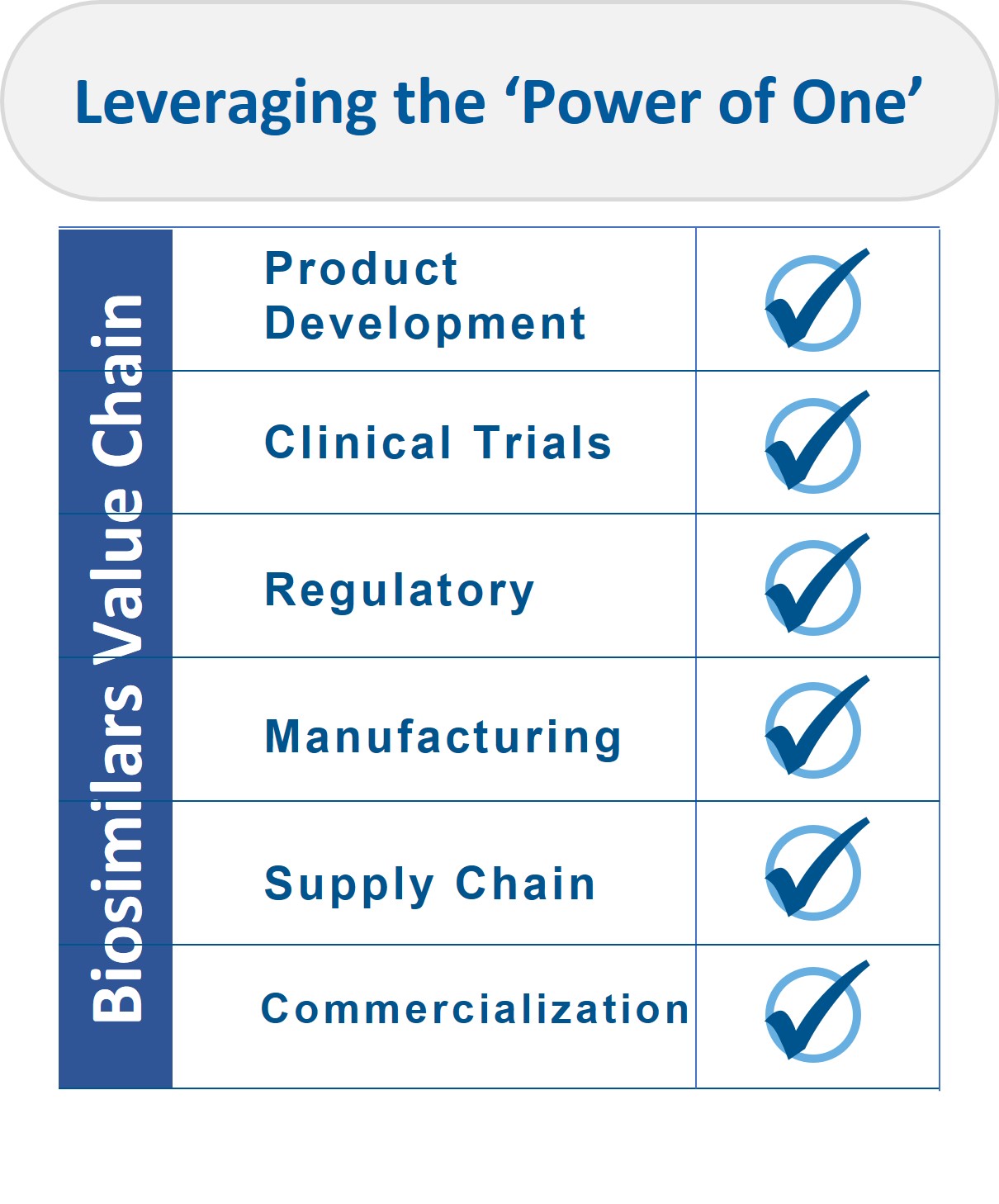
The integration of Viatris’ acquired global biosimilars business and Biocon Biologics’ existing capabilities in research and development, global scale manufacturing and commercialization in several emerging markets positions Biocon Biologics as a biosimilars organization with ‘end-to-end’ capabilities.
With the closing of the deal, Biocon Biologics has full ownership of its collaboration assets with Viatris, Trastuzumab, Pegfilgrastim, Bevacizumab, Insulin Glargine 100U, Insulin Aspart, Pertuzumab and Insulin Glargine 300U, as well as Viatris’ rights for the in-licensed immunology products of Adalimumab and Etanercept. Biocon Biologics has also acquired Viatris’ rights for Aflibercept, which is used to treat several ophthalmology conditions. Viatris has been the ‘first to file’ Aflibercept in the U.S.
Effective from the date of closing of the deal on November 29, 2022, Biocon Biologics has started recognizing the full value of revenue and associated profits of the acquired business, a step-up from the earlier arrangement.
This acquisition also provides Biocon Biologics greater agility in decision-making and helps improve operational efficiencies in supply chain, capital allocation and distribution.
With this acquisition Biocon Biologics emerges as a world leading biosimilars player.
“The completion of the acquisition of Viatris’ global biosimilars business is a historic inflection point in Biocon Biologics’ journey of becoming a world leading, fully integrated biosimilars enterprise, committed to serve patients’ needs for affordable access to essential biomedicines. It will fast-track our direct entry into several advanced and emerging markets. This acquisition builds on our decade-long partnership and will enable us to realize our vision of addressing global health inequities. I believe this move will strengthen our value proposition to deliver long-term value to Biocon and Biocon Biologics’ shareholders.”
Kiran Mazumdar-Shaw
Executive Chairperson, Biocon & Biocon Biologics.
Power of One
Enabling Equitable Access to Affordable Healthcare
Financial Details of the Transaction
Biocon Biologics has issued Compulsorily Convertible Preference Shares (CCPS) in the Company valued at USD 1 billion, equivalent to an equity stake of at least 12.9% on a fully diluted basis, and made an upfront cash payment of USD 2 billion to Viatris.
To fund the upfront payment, Biocon Biologics has secured USD 1.2 billion of Sustainability Linked Loan (SLL). The balance has been funded through an equity infusion of USD 650 million by Biocon Limited and USD 150 million by Serum Institute Life Sciences (SILS).
Rajiv Malik, President & Director of Viatris, has joined the Board of Biocon Biologics Ltd as Non-Executive, Non-Independent and Nominee Director of Viatris Inc. Rajiv Malik is a well-regarded business leader with over 40 years of experience in the global pharma industry.

“We have a rich legacy of challenging the status quo, breaking barriers and now with the acquisition of Viatris’ global biosimilars business, we Dare to Dream Big! This is undoubtedly the most defining time in our history, a game-changing event that catapults Biocon Biologics into the big league – a unique, fully integrated leading global biosimilars company.”
Shreehas Tambe
CEO & Managing Director, Biocon Biologics
Ensuring Smooth Transition
To ensure seamless business continuity and allow for a gradual integration of people and business activities, Biocon Biologics has drawn up a comprehensive global integration plan and has started migrating country-wise business operations in a phased manner.
Viatris continues to provide commercial and other transition services to Biocon Biologics as part of a pre-agreed Transition Services Agreement.
In parallel, Biocon Biologics is designing a bespoke country-specific strategy and business model that optimizes for revenues and profitability, creating value for all our stakeholders.
Investing in the Future
Biocon and Viatris have enjoyed a long and mutually beneficial partnership since 2009. Together, we invested ahead of time in R&D, manufacturing scale and capability building, for biosimilars. Together, we achieved many global ‘firsts’ and set new benchmarks for the global biosimilars industry.
With this acquisition, we are well placed to achieve our ambition of becoming a global biosimilars leader.
Our 40-year legacy of being on the cutting edge of science and technology has enabled us to build a unique portfolio of 20 biosimilar assets, including insulins and monoclonal antibodies spanning therapy areas such as diabetes, oncology, immunology and ophthalmology. With one of the broadest and deepest biosimilar portfolios in the industry, Biocon Biologics can make a meaningful difference to patients’ lives and deliver significant savings to healthcare systems around the world.
We also have significant global scale manufacturing capacity dedicated to biosimilars. This acquisition helps us build deeper capabilities, robust supply chains and stronger leadership by combining the complementary capabilities and strengths of two long-term partners. It allows us to leverage THE POWER OF ONE to reduce healthcare inequities and transform healthcare, worldwide.
 Brazil
Brazil Egypt
Egypt Europe
Europe Hong Kong
Hong Kong Malaysia
Malaysia Mexico
Mexico Morocco
Morocco Philippines
Philippines Saudi Arabia
Saudi Arabia South Africa
South Africa Taiwan
Taiwan Thailand
Thailand Tunisia
Tunisia Turkey
Turkey UAE
UAE USA
USA Vietnam
Vietnam Global HQ
Global HQ